Health Care Roundup: Drug Pricing Plan, 2019 Premium Increases, Short-Term Plans, Medicare Mulls Covering Gene Therapies, and More
Your feedback is always welcome to make our content more useful to you. Please send comments to feedback@canceradvocacy.org.
Subscribe to our email list and receive these updates in your email box each week »
HEALTH CARE HIGHLIGHTS
Trump Administration Promotes a Drug Pricing Plan
On Friday, May 11, 2018, President Trump announced a drug pricing blueprint to address prescription drug prices. Initial media coverage of the plan suggested that the blueprint failed to honor the President’s campaign commitment to negotiation of drug prices. Overall, the press and policymaker reaction to the plan was muted, with some suggesting that the plan would have limited impact. Although details of the plan are still unclear, certain elements of the plan may have an impact on how cancer patients receive and pay for their prescription drugs. There may be changes in the coverage and payment for both intravenous, physician-administered cancer drugs and oral, self-administered cancer therapies. The public has 60 days to comment on the drug pricing plan through the “Request for Information” released by the Trump Administration. NCCS will evaluate the direct patient impact of the plan provisions that relate to coverage and payment for cancer drugs and will outline the issues of concern to cancer patients.
Insurance Premium Increases for 2019, the Causes, and Efforts to Lower Them
Insurers have begun to project next year’s premiums for plans offered through the Affordable Care Act (ACA) marketplaces. The premiums that have been announced to date for 2019 are significant increases over premiums in 2018. Insurance companies signaled that adjustments to the premiums are possible if they receive relief from the costs associated with insuring expensive patients. Members of Congress are blaming each other for the premium increases. Congressional Democrats contend that the actions of the Trump Administration to undermine the ACA enrollment process and to advance short-term, limited-duration plans will further undermine the ACA marketplaces. The Trump Administration and some Congressional Republicans maintain that the ACA is fundamentally flawed and that repeal is still necessary. The Hill has reported that rate increases have reignited the “Obamacare war.” In fact, Senator Lindsay Graham is reportedly working on a “new” Obamacare repeal bill.
Short-Term Plans: Enrollment will be Significant and Negative Effects on Marketplace will be Substantial
The Trump Administration’s proposed expansion of short-term plans will reportedly be finalized by June 2018. These plans, sometimes called “skinny” or even “junk” plans, will not be required to meet ACA standards for benefits, cost-sharing, guaranteed issue, and pre-existing condition protections. The Administration has touted these plans as an affordable insurance option for healthy adults and has suggested that uptake of the plans will be slow and the impact on the ACA health insurance marketplaces will be modest.
This week, the Actuary of the Centers for Medicare & Medicaid Services (CMS), who is independent from the CMS leadership, projected that 1.8 million Americans would elect short-term coverage, with 800,000 coming from the ACA marketplace. This projection means that almost 2 million Americans will have only short-term coverage of limited scope and significant cost-sharing burden and that the diversion of hundreds of thousands from the marketplace will destabilize the ACA marketplace. For cancer patients who rely on ACA coverage, premiums will increase, potentially substantially, if they are not eligible for premium subsidies. The expansion of short-term plans will also cost the federal government $38.7 billion over ten years with increased expenditures for premium subsidies, the CMS Actuary reported.
CHART OF THE WEEK
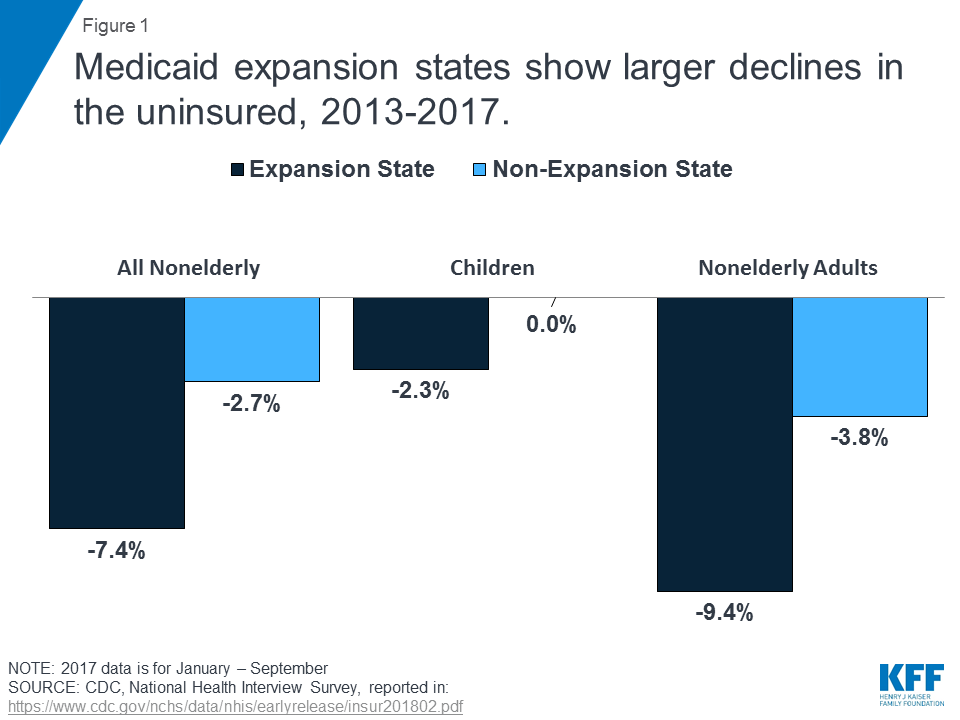
A Kaiser Family Foundation report entitled, “Implications of the ACA Medicaid Expansion: A Look at the Data and Evidence,” reported that “Medicaid expansion states experienced significant coverage gains and reductions in uninsured rates compared to non-expansion states for the low-income population broadly.”
IMPORTANT READS
Healthy America, a big new plan to combine Medicaid and Obamacare, explained
Via Vox.com
The Urban Institute, a think tank in Washington, DC, has acknowledged the deep divide about the ACA and the renewed push to repeal the ACA. They have proposed an alternative that protects employer-based insurance and Medicare and creates a new system for individual and families seeking health insurance coverage. Premiums in the system would be income-based and premium subsidies would be available for those who need them. The plan would also impose limits on provider payments and other cost controls would be implemented to control premium costs. The Urban Institute plan includes a chart that compares a wide range of health care reform proposals, identifying the strengths and weaknesses of each.
More men with low-risk prostate cancer are forgoing aggressive treatment
Via Washington Post
In a study published in the Journal of the American Medical Association (JAMA), researchers reported that men with low-risk prostate cancer are increasingly choosing active surveillance of their disease instead of surgery and radiation. The study, which included more than 125,000 men with prostate cancer treated between 2005 and 2015, found a major shift in treatment decisions over that decade. In 2005, only 27 percent of men under age 65 chose watchful waiting instead of active treatment. By 2015, that proportion had grown to 72 percent. Cancer care experts are applauding the shift in treatment choices, which spares men the serious side effects of treatment and results in comparable outcomes.
CMS considers paying all Medicare providers for cancer gene therapies
Via Modern Healthcare
The Centers for Medicare & Medicaid Services (CMS) has initiated a review of how and under what terms the Medicare program will cover the recently approved CAR T-cell therapies. These therapies have provided significant benefits to certain leukemia and lymphoma patients. Some patients experience serious side effects from the treatments. Public comment on the review is accepted until June 15, 2018. A public meeting will be held on August 22, 2018. The final decision is due on February 16, 2019.



