2020 State of
Cancer Survivorship
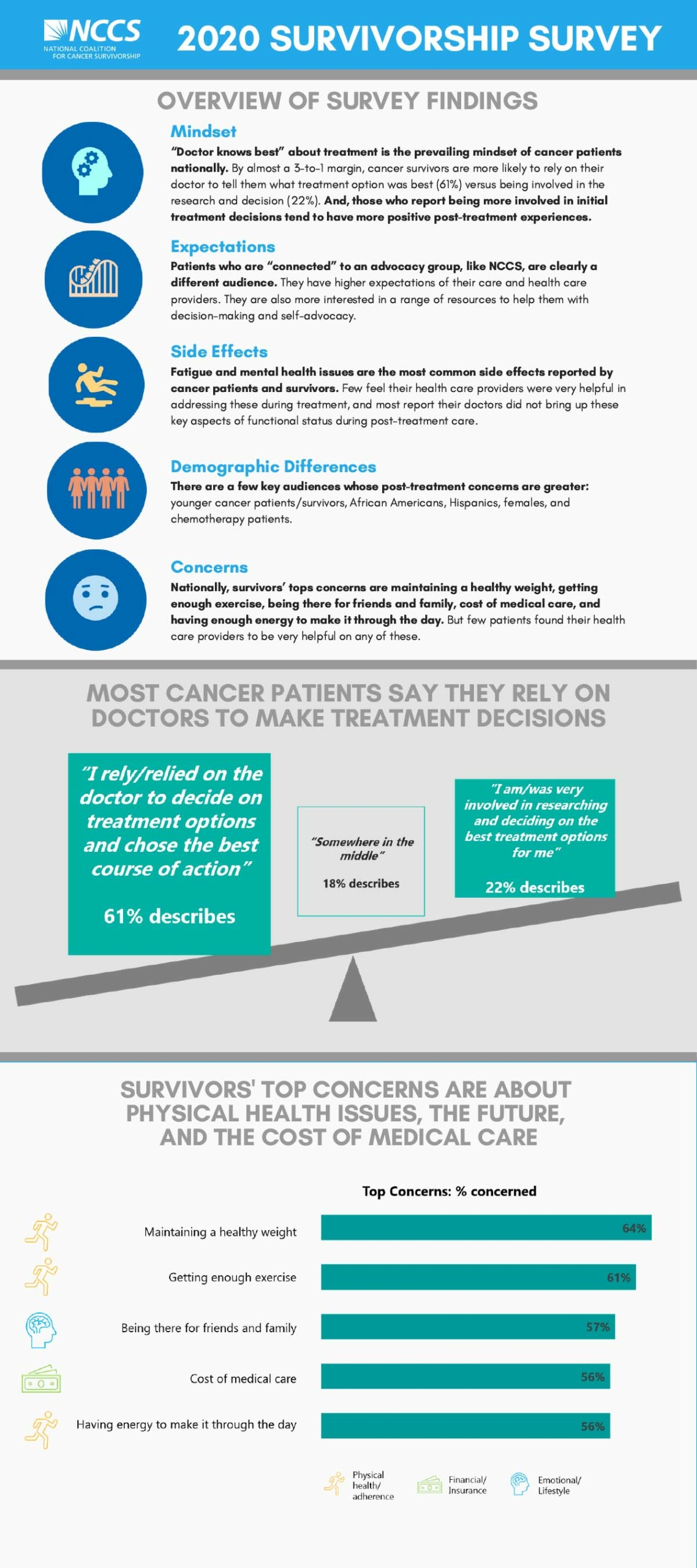
NCCS conducted the 2020 State of Cancer Survivorship Survey with a broad, national sample that mirrors the population of cancer survivors in the United States. We found consistent themes from the first NCCS survivorship survey in 2018, such as: cancer survivors face significant challenges both during and after treatment. While the cancer care team effectively addresses many of the physical effects of cancer treatment, survivors reported that their care team is not as helpful addressing other effects of their cancer, including fatigue, anxiety, and depression.
When we compare the national sample with patients who are connected to an advocacy organization, such as NCCS, we see differences in the level of empowerment and involvement in decision-making. The majority of patients in the national sample defer to their clinicians for decision-making about their care.
Educating and empowering patients is an important goal of NCCS, but at the same time, we must work toward a health care system that works for ALL patients, even if they are not able or interested in playing an active role in their care.
NCCS CEO Shelley Fuld Nasso
Watch the Web Briefing
In this briefing, you will:
- Listen to NCCS CEO Shelley Fuld Nasso and Edge Research Principal Pam Loeb, who will present the survey findings.
- Hear what cancer survivors reported about how helpful their health care teams are at addressing a range of side effects.
- Learn more about cancer survivors’ experiences and needs for post-treatment information and care, as well as the demand for survivorship resources.
- Better understand the patient experience, including new questions about cancer survivors’ mindset, the multi-disciplinary team, clinical trials, and the help needed to address side effects.
To try to address these gaps, we encourage you to become involved in NCCS and use these resources.
Access the 2018-2019 NCCS Survivorship Survey »
Want to share your survivorship journey?
Are you, or someone you know, advocating for care? Share your story.
This program is supported by an educational grant from Bristol-Myers Squibb.